
Last Updated on June 15, 2015
Advancing Biomarkers to Screen and Diagnose Preeclampsia: A Report to Stakeholders
In December 2012, the Preeclampsia Foundation convened stakeholders from industry, academia, FDA, laboratories, patients and clinicians to assess the state of biomarkers for preeclampsia, identify barriers and brainstorm strategies to overcome those challenges. This report includes background on the state of molecular biomarkers for preeclampsia screening and diagnosis, identification of the needs and challenges facing the field, the proceedings and participants of the Biomarkers Consortium, and the resulting calls to action, broken out by stakeholders (research and academia, manufacturers and laboratories, clinical, regulatory, and patient organizations).
Read the complete report of the first Biomarkers Consortium.
Related Articles

Nurses play a vital role in detecting preeclampsia and caring for patient before, during, and beyond pregnancy.

A key component needed in the fight against preeclampsia is the development of tests for simple, rapid, and accurate diagnosis and prediction through the development and adoption of biomarkers.
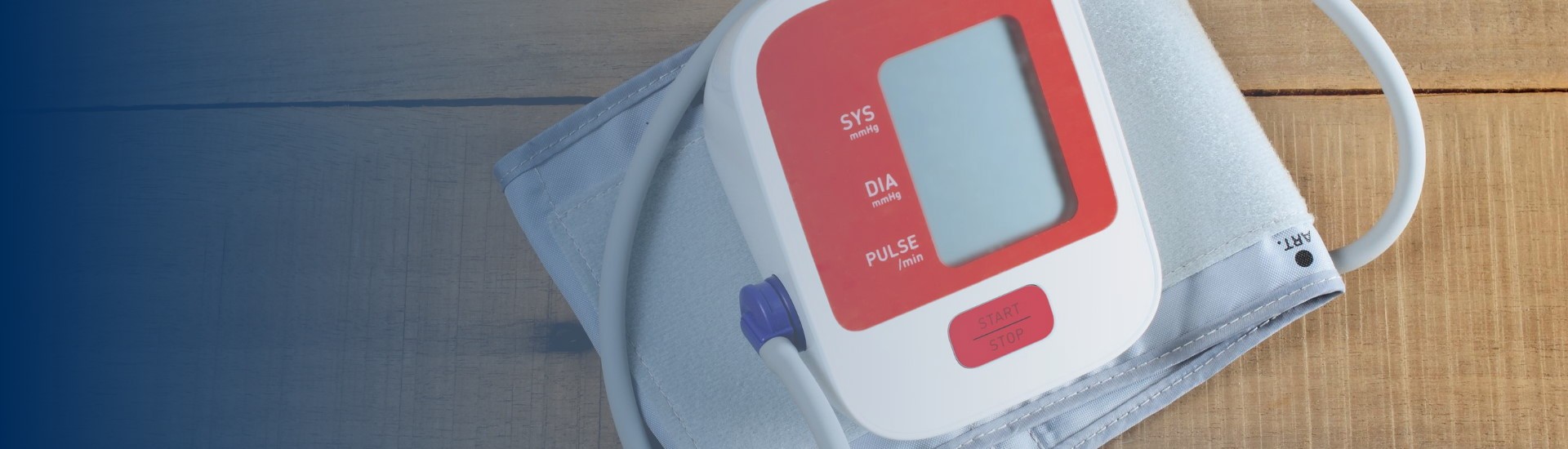
As a first step to address the need for self-monitored blood pressure, the Preeclampsia Foundation started providing the Cuff Kit™ in June 2020 to women at highest risk of developing preeclampsia and...
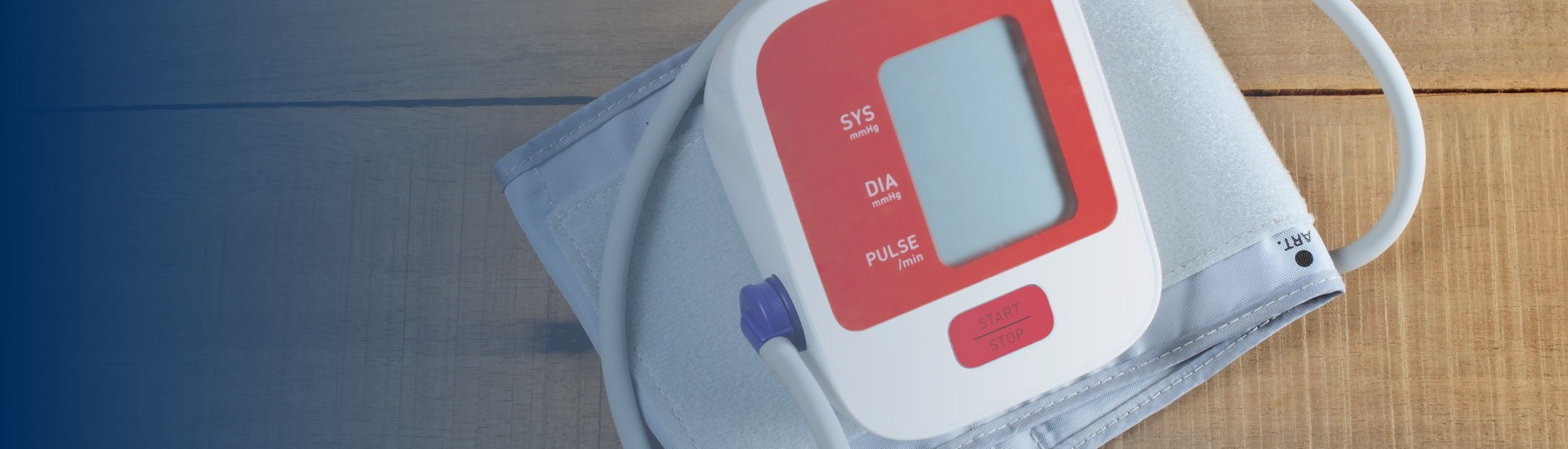
Every woman should be able to check her own blood pressure at home.
Order our Ask About Aspirin Rack Card. Aspirin can prevent the formation of blood clots. This can make aspirin useful in treating or preventing some conditions like heart attacks and st...

Washington, DC – April 11, 2024 – On April 10, one day before the start of Black Maternal Health Week, the Preeclampsia Foundation in partnership with Thermo Fisher Diagnostics held a Hill...

Recently, I came across a social media post calling attention to the global maternal health crisis from a Black woman’s perspective. Someone responded to the post asking, “What’s rac...

The Preeclampsia Foundation announced today that the Centers for Disease Control and Prevention (CDC) awarded new funding for its MoMMA’s Voices program, a national coalition of patient advocacy...

The U.S. Preventive Services Task Force (USPSTF) released today a final recommendation statement on screening for hypertensive disorders of pregnancy. The Task Force recommends that all pregnant peopl...

September 7, 2023 - Rockville, Md. — The U.S. Department of Health and Human Services (HHS)—through the Health Resources and Services Administration (HRSA)— awarded an evaluation a...
